Compromise no more
Years from now, scientists will marvel at the fact that Foetal Bovine Serum (FBS) took so long to be replaced by a chemically defined alternative. But don't just take our word for it. Here are the facts that convinced us it was time to solve this problem, for once and for all.
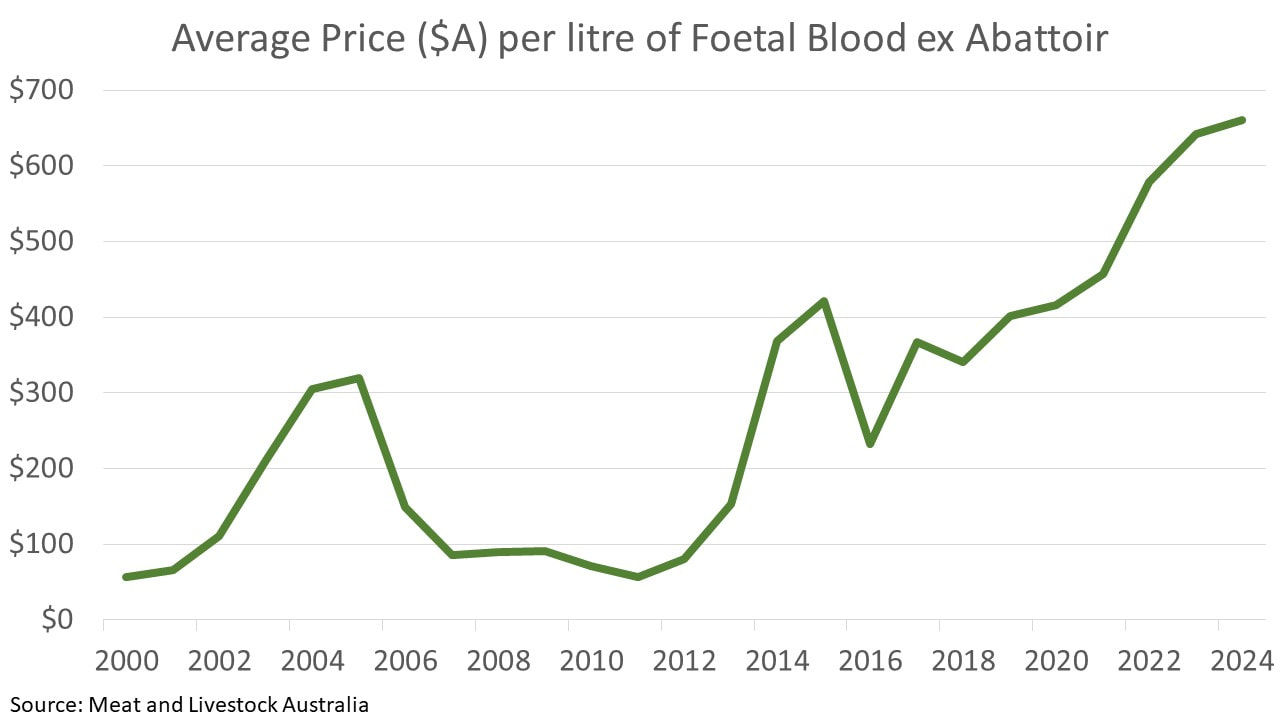
Say goodbye to price volatilityFoetal Bovine Serum is subject to extreme price volatility. Climatic variation and disease significantly influence supply. Further, widespread adoption of cow pregnancy testing is steadily reducing the supply of FBS. This is placing upward pressure on prices which have increased sixfold since 2000.
When you use Foetal Bovine Serum Replacement Solution (FRS™️), you enjoy the peace of mind that our prices are not subject to these market pressures. |
Consistency in research tools is critical to ensuring study reproducibility
|
Cows have diverse characteristics, leading to differences between batches of Foetal Bovine Serum. This is a known problem, sometimes leading labs to stock-pile particular batches of Foetal Bovine Serum for future research. Sometimes, this is an unknown problem that challenges reproducibility in scientific studies - or even the conclusions of the results. Case studies into how Foetal Bovine Serum variation may be impacting your scientific results were recently published in both Science and Nature.
Our FRS™️ formulations are chemically defined and consistent between batches, offering researchers confidence in their reproducibility and reliability. The expensive days of stockpiling batches are over. |
“Interlaboratory reproducibility becomes crucial when in vitro methods are used in applied research, such as preclinical studies with human cells, or for regulatory safety testing of pharmaceuticals and chemicals.”
(van der Valk, 2022)
(van der Valk, 2022)
Regulatory bodies are increasingly encouraging clinical therapies to avoid Foetal Bovine Serum (FBS) due to known contaminant and reproducibility challenges
|
If you’re performing academic research with the aim of having your findings translated to the clinic, then moving away from the use of Foetal Bovine Serum is essential.
Foetal Bovine Serum is generally unsuitable for human clinical application. Key regulatory bodies such as the FDA and EMA discourage its use due to inconsistencies between batches and the presence of contaminants. In cases when 'clinical grade' Foetal Bovine Serum is available, it is extremely expensive, subject to significant price volatility and continues to suffer from batch inconsistency. Cell based therapies involve the expansion of primary cells ex-vivo prior to their use as a therapeutic. They are a revolutionary new field of medicine currently hampered by the high cost of cell culture media. FRS™️ provides a safe, clean and cost-effective alternative to clinical grade Foetal Bovine Serum. |
FRS supports the growth of a broad range of particularly demanding cell types, including primary cellsAlternatives to Foetal Bovine Serum have been historically characterised by poor proliferation performance, poor longevity and unsuitability for a sufficient breadth of cell types.
At Media City Scientific, we are rapidly overcoming these challenges with FRS™️ offering similar or superior performance to Foetal Bovine Serum across these key metrics. This means you only need one solution for all of the cells growing in your lab. |
Into the future...
FBS is affecting your research resultsThis is an inconvenient research truth that many scientists are insufficiently aware of.
In this study, mouse immune cells were cultured in the presence of eight different animal serums. The immune cell response varied dramatically. Mouse cells cultured with mouse serum produced an immune response that resembled a mouse. Mouse cells cultured with human serum produced an immune response more resembling a human. Media City Scientific envisages a future world in which scientists are not using human cells cultured in Foetal Bovine Serum to make early stage discoveries supposedly relevant to human health. Instead, they use chemically defined FRS™️ products that deliver similar results to culturing human cells in human serum. |
Frequently Asked Questions
Does FRS™️ support the growth of a wide range of cell types?
Yes, FRS™️ is a broad spectrum chemically defined replacement to Foetal Bovine Serum. To date, we have tested FRS™️ on a wide range of primary cell types known to have particularly demanding needs.
Yes, FRS™️ is a broad spectrum chemically defined replacement to Foetal Bovine Serum. To date, we have tested FRS™️ on a wide range of primary cell types known to have particularly demanding needs.
Does FRS™️ vary between batches? No, each product line of FRS™️ is the same whether you purchased it last year, this year or in ten years' time. This enables unparalleled reproducibility and inter-laboratory collaboration - even globally.
Surely FRS™️ uses some animal components? No, FRS™️ is chemically defined and animal component free. This makes FRS™️ contaminant free and ideal for use in clinical settings.
Will it be challenging to adapt my cells to FRS™️? FRS™️ is designed for direct adaptation of cells from Foetal Bovine Serum to FRS™️ rather than multi-step sequential adaptation over several weeks.
Wow this sounds great, but there has to be a catch - it's super expensive, right?
Nope! Whilst other cell-specific, chemically defined replacements to Foetal Bovine Serum are extremely expensive, the price of broad spectrum FRS™️ is on par with that of Foetal Bovine Serum.
Nope! Whilst other cell-specific, chemically defined replacements to Foetal Bovine Serum are extremely expensive, the price of broad spectrum FRS™️ is on par with that of Foetal Bovine Serum.